Description
Global Molecular Oncology Diagnostics Market: Industry Overview
The global molecular oncology diagnostics market was valued at $3,620.6 million in 2021 and is anticipated to reach $12,130.5 million by 2032, witnessing a CAGR of 11.43% during the forecast period 2022-2032. The growth in the global molecular oncology diagnostics market is expected to be driven by the rising prevalence of cancers and increased transformations in biomarker identification.
Market Lifecycle Stage
Molecular oncology diagnostics play an essential role in the diagnosis and prognosis of different types of cancers and in improving patient outcomes. Over the last decade, cancer treatment and diagnostics has become a major public health concern across the globe.
With over 1.5 million instances reported each year, diseases like cancer can have far-reaching implications for individuals as well as a greater influence on the socioeconomic strata.
The market for appropriate testing assay kits is expanding rapidly, owing to the increased importance of early diagnosis to minimize further hazards, especially with rates on the rise. The rising global cancer incidence and the growing demand for early detection are expected to drive the growth of the global molecular oncology diagnostics market during the forecast period.
This expanded usage of kit and assay, instrument, and software has enabled end customers to describe particular system requirements, prompting suppliers to design more appealing and practical products and molecular diagnostics choices.
Impact
• The presence of major kit and assay providers of molecular oncology diagnostics has a major impact on the market. For instance, in March 2021, Biocartis NV launched Idylla GeneFusion Assay as a Rapid Lab Workflow Solution for gene fusion testing. This product provides a much faster testing solution for laboratories in comparison to other testing methods, including NGS.
• Companies such as Bio-Rad Laboratories, Inc., in August 2022, acquired Curiosity Diagnostics. The company acquired all the outstanding shares of the latter from Scope Fluidics, S.A. for its growth in the medical diagnostic and healthcare markets, for a total consideration of up to $170 million. Similarly, in February 2022, Becton and Dickinson Company (BD) acquired Cytogons to gain exclusive access to advanced assays licensed from the EuroFlow Consortium and strengthen BD’s leadership position in the molecular oncology diagnostics segment.
Impact of COVID-19
Cancer screening is crucial for early cancer detection; however, COVID-19 slowed the cancer screening infrastructure greatly. Many cancer organizations have championed the idea of suspending cancer screening services to patients to alter the provision of health care resources.
According to the declaration of a national emergency in the U.S. on March 13th, 2020, organizations such as the American Cancer Society advised people to put their cancer screening plans on hold until further notice during the COVID-19 outbreak. Cancer screening services have been severely disrupted because of this proposal, as well as other contextual circumstances (e.g., social exclusion measures).
In response to the COVID-19 pandemic and the impact on medical research in the U.S., the National Institutes of Health (NIH) and the National Cancer Institute (NCI) both extended grant application deadlines, laid back reporting requirements, and offer flexibility in how to grant money is spent.
COVID-19 drastically reduced fundraising possibilities for cancer-focused charitable research organizations, which provide up to half of all cancer research funding in the U.S. and frequently fund high-risk, high-reward projects even though the national government supported research work and offered medical researchers the flexibility to implement their skill set and knowledge to studying SARSCoV-2.
Market Segmentation:
Segmentation 1: by Product
• Kits and Assays
• Instruments
• Software
Based on products, the global molecular oncology diagnostics market is expected to be dominated by kit and assay. This is due to the high number of applications performed by kits and their ease of integration with other systems.
Segmentation 2: by Technology
• Next-Generation Sequencing (NGS)
• Polymerase Chain Reaction (PCR)
• Immunohistochemistry (IHC)
• Fluorescence In-Situ Hybridization (FISH)
• Flow Cytometry
• Other Technologies
Based on technology, the global molecular oncology diagnostics market is dominated by polymerase chain reaction (PCR). However, next-generation sequencing (NGS) is expected to be the fastest-growing segment in the market. This is because PCR techniques play an important role in targeted NGS sequencing because they allow for the simultaneous production of several NGS libraries and the sequencing of numerous targeted regions.
Segmentation 3: by Application
• Clinical Diagnostic
• Research Use
Based on applications, the global molecular oncology diagnostics market is expected to be dominated by the clinical diagnostic segment. This is due to the high usage of technologies such as companion diagnostics and liquid biopsy emerge.
Segmentation 4: by Cancer Type
• Solid Tumor
o Breast Cancer
o Lung Cancer
o Colorectal Cancer
o Prostate Cancer
o Ovarian Cancer
o Other Solid Tumors
• Hematological Malignancy
o Lymphoma
o Leukemia
o Multiple Myeloma
o Other Hematological Malignancies
Based on cancer type, the global molecular oncology diagnostics market is dominated by solid tumor cancer type, where breast cancer is expected to be the leading segment. The growth of advanced solid tumor testing has mostly focused on the treatment of distinct cancer kinds, indications, and indication subtypes. The hematological malignancy segment is expected to show a fast growth rate during the forecast period.
Segmentation 5: by End User
• Hospitals and Diagnostic Centers
• Reference Laboratories
• Pharmaceutical and Biotechnology Companies
• Academic and Research Institutes
Based on end users, global molecular oncology diagnostics was dominated by the hospitals and diagnostic centers segment. The growth of this segment is primarily attributed to the high use of molecular diagnostic tests by hospitals in these fields.
Segmentation 6: by Region
• North America
• Europe
• Asia-Pacific
• Rest-of-the-World
North America dominated the global market with a revenue of $1,588.6 in 2021. However, the Asia-Pacific region, constituting several emerging economies, is expected to register the highest CAGR of 12.60% during the forecast period 2022-2032.
Recent Developments in the Global Molecular Oncology Diagnostics Market
• In August 2022, Guardant Health expanded its Guardant Reveal usage, a liquid biopsy test for residual disease detection and recurrence monitoring, to include early-stage breast and lung cancers. It is the only tissue-free test used for colorectal cancer (CRC) and is now available for patients with breast and lung cancer.
• In December 2021, QIAGEN partnered with Denovo Biopharma to develop a companion diagnostic test for the treatment of diffuse large B-cell lymphoma. This test identified patients expressing Denovo genomic marker 1 (DGM1), which responded to Denovo’s investigational cancer drug DB102.
• In September 2021, Illumina partnered with Merck to develop and commercialize research tests for use in identifying specific cancer genetic mutations used in the assessment of homologous recombination deficiency (HRD).
• In August 2021, Agilent Technologies, Inc. expanded its CE-IVD marked PD-L1 IHC 22C3 pharmDx assay usage in Europe. This assay was used as an aid in identifying esophageal cancer patients for treatments with KEYTRUDA using a combined positive score.
Demand – Drivers and Limitations
Following are the demand drivers for the global molecular oncology diagnostics market:
• Rising Incidence of Cancer Cases
• Launch of Innovative Products in Molecular Oncology Diagnostics Ecosystem
• Growth in Biomarker identification and Transformations in Molecular Techniques
The market is expected to face some limitations due to the following challenges:
• Lack of Qualified Professionals
• Opaque Regulatory Framework Delaying the Approval of New Molecular Diagnostic Tests
• High Cost of Equipment Hindering the Adoption Rate
How can this report add value to an organization?
Product/Innovation Strategy: The report considers only product-based companies. The industry is witnessing constant development and product launches with new and innovative upgrades. Additionally, emerging technologies like microarray are also expected to be integrated more into molecular oncology diagnostics soon, which is expected to further boost market growth.
Growth/Marketing Strategy: The key components in molecular oncology diagnostics are the kit and assay, instrument, and software. The cost of the kit and assay depends on the number of integrations one wants the system with. However, the software charges usually surpass the cost of instruments and kits and assays that are necessary to ensure the purchase of the right type of instruments and correct integration and placements.
Competitive Strategy: The key players in the global molecular oncology diagnostics market analyzed and profiled in the study consist of product-based companies. Moreover, a detailed competitive benchmarking of the players operating in the global molecular oncology diagnostics market has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations are expected to aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts, analyzing company coverage, product portfolio, and market penetration.
Some of the prominent names in this market are:
• Agilent Technologies, Inc.
• Abbott.
• Biocartis NV
• Bio-Rad Laboratories, Inc.
• F. Hoffmann-La Roche Ltd.
• QIAGEN N.V.
• Thermo Fisher Scientific, Inc.
• Danaher.
• Guardant Health
• HTG Molecular Diagnostics, Inc.
• Illumina, Inc.
• Invivoscribe, Inc.
• Myriad Genetics, Inc.
• Sysmex Corporation
Related Report: Flow Cytometry in Oncology and Immunology Market – A Global Market and Regional: Focus on Offering, Technology, End User, Type, Application, and Country – Analysis and Forecast, 2022-2032
Related Report: Oncology Precision Medicine Market – A Global and Regional Analysis: Focus on Ecosystem and Application – Analysis and Forecast, 2020-2031
Related Report: Global Immuno-Oncology Clinical Trials Market Analysis and Forecast Report 2030
Related Report: Interventional Oncology Devices Market – A Global and Regional Analysis: Focus on Cancer Type, Product Type, End Users, and Country-Wise Analysis – Analysis and Forecast, 2021-2030
Related Report: Companion Diagnostics for Oncology Market : Global Analysis of Market Size, Share & Trends for 2019 – 2020 and Forecasts to 2030
Related Report: Global Radiation Therapy in the Oncology Market: Focus on Radiation Therapy Systems, Product Regulation, Key Strategies and Developments, Market Dynamics, 15 Company Profiles, and 12 Countries Data and Cross Segmentation – Analysis and Forecast, 2021-2031
Related Report: Hospital EMR Systems Market: Market Segments: By Product Type (Inpatient EMR and Outpatient EMR); By Component (Software and Services); By Application (Cardiology, Neurology, Radiology, Oncology and Others); By End-User (Hospital-based EMR and Physician-based EMR); and Region – 2Global Analysis by Market Size, Share & Trends for 2014 – 22020 and Forecasts to 2030
Related Report: Global Total Fluid Management Market: Market Segments: By Product Type [Infusion Therapy Products (Infusion Devices, IV Access devices, IV Solutions and Products), Renal Products (In-Center Hemodialysis Products, Home Hemodialysis (HHD) Products, Peritoneal dialysis Products, Acute Dialysis Products), Endoscopy Fluid Management Products]; By Application (Urology, Cardiology, Orthopedic/Osteology, Neurology, Oncology, Gastroenterology, Others); By End-user (Hospitals, Clinics, Dialysis Centers, Home Care Settings); and Region – 2Analysis of Market Size, Share & Trends for 2014 – 22019 and Forecasts to 2030
Related Report: Global Next-Generation Antibody Therapeutics Market: Market Segments: By Therapeutic Area (Oncology Autoimmune/Inflammatory); By Technology (Antibody-Drug Conjugates (ADCs), Bispecific Antibodies, Fc-engineered Antibodies, Antibody Fragments and Antibody-like Proteins & Biosimilar Antibody Products);and Region – 2Analysis of Market Size, Share & Trends for 2014 – 22019 and Forecasts to 2030
Related Report: Global Flow Cytometry in Oncology Market: Focus on Product Type, Technology, Type of Cancer, Applications, End Users, Country Data (16 Countries), and Competitive Landscape – Analysis and Forecast, 2021-2031
Related Report: Global Companion Diagnostics for Oncology Market Research Report – Forecast till 2027
Table of Contents
1 Market
1.1 Product Definition
1.2 Inclusion and Exclusion
1.3 Scope of the Study
1.4 Key Questions Answered in the Report
2 Research Methodology
2.1 Global Molecular Oncology Diagnostics Market: Research Methodology
2.2 Primary Data Sources
2.3 Secondary Data Sources
2.4 Market Estimation Model
2.5 Criteria for Company Profiling
3 Global Molecular Oncology Diagnostics Market: Overview
3.1 Market Overview
3.1.1 Evolution of Molecular Cancer Diagnostics and Current Status
3.2 Global Molecular Oncology Diagnostics Market Size and Forecast (2021-2032)
3.3 Global Incidence and Prevalence of Cancer (by Type)
3.3.1 North America
3.3.1.1 Solid Tumor
3.3.1.2 Hematological Malignancy
3.3.2 Europe
3.3.2.1 Solid Tumor
3.3.2.2 Hematological Malignancy
3.3.3 Asia-Pacific
3.3.3.1 Solid Tumor
3.3.3.2 Hematological Malignancy
3.4 Impact of COVID-19 on the Molecular Oncology Diagnostics Market
3.4.1 Impact on Market Size
3.5 Liquid Biopsy-Based Cancer Molecular Diagnostics
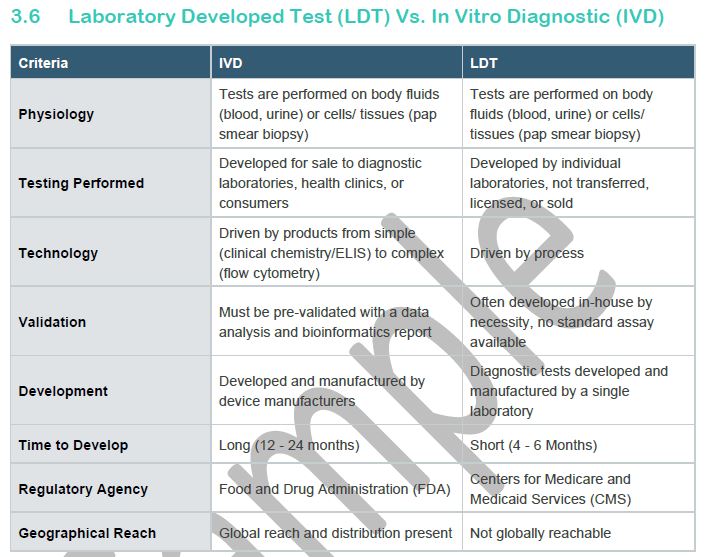
3.6 Laboratory Developed Test (LDT) Vs. In Vitro Diagnostic (IVD)
3.7 Role of Companion Diagnostics in the Molecular Oncology Diagnostics Market
4 Global Molecular Oncology Diagnostics Market: Industry Analysis
4.1 Legal Requirements and Framework in the U.S.
4.1.1 FDA Regulation
4.1.2 CMS Regulation (Reimbursement Scenario)
4.2 Legal Requirements and Framework in Europe
4.3 Legal Requirements and Framework in Asia-Pacific
4.3.1 China
4.3.2 Japan
5 Market Dynamics
5.1 Overview
5.1 Impact Analysis
5.2 Growth Drivers
5.2.1 Rising Incidence of Cancer Cases
5.2.2 Launch of Innovative Products in Molecular Oncology Diagnostics Ecosystem
5.2.3 Growth in Biomarker identification and Transformations in Molecular Techniques
5.3 Growth Restraints
5.3.1 Lack of Qualified Professionals
5.3.2 Opaque Regulatory Framework Delaying the Approval of New Molecular Diagnostic Tests
5.3.3 High Cost of Equipment Hindering the Adoption Rate
5.4 Growth Opportunities
5.4.1 Partnerships and Collaborations between Various Healthcare Stakeholders
5.4.2 Upsurge of Next-Generation Ultrasensitive Molecular Diagnostics
6 Competitive Landscape
6.1 Mergers and Acquisitions
6.2 Synergistic Activities
6.3 Product Launch and Approval Activities
6.4 Expansion, Insurance, and Other Key Developments
6.5 Market-Share Analysis
6.6 Growth-Share Analysis
7 Global Molecular Oncology Diagnostics Market (by Product), $Million, 2021-2032
7.1 Overview
7.2 Kits and Assays
7.3 Instruments
7.4 Software
8 Global Molecular Oncology Diagnostics Market (by Technology), $Million, 2021-2032
8.1 Overview
8.2 Polymerase Chain Reaction (PCR)
8.3 Next-Generation Sequencing (NGS)
8.4 Immunohistochemistry (IHC)
8.5 Fluorescence In-Situ Hybridization (FISH)
8.6 Flow Cytometry
8.7 Other Technologies
9 Global Molecular Oncology Diagnostics Market (by Application), $Million, 2021-2032
9.1 Overview
9.2 Clinical Diagnostic
9.3 Research Use
10 Global Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
10.1 Overview
10.1.1 Solid Tumor
10.1.1.1 Breast Cancer
10.1.1.2 Lung Cancer
10.1.1.3 Colorectal Cancer
10.1.1.4 Prostate Cancer
10.1.1.5 Ovarian Cancer
10.1.1.6 Other Solid Tumors
10.1.2 Hematological Malignancy
10.1.2.1 Lymphoma
10.1.2.2 Leukemia
10.1.2.3 Multiple Myeloma
10.1.2.4 Other Hematological Malignancies
11 Global Molecular Oncology Diagnostics Market (by End User), $Million, 2021-2032
11.1 Overview
11.2 Hospitals and Diagnostic Centers
11.3 Reference Laboratories
11.4 Pharmaceutical and Biotechnology Companies
11.5 Academic and Research Institutes
12 Region
12.1 Overview
12.2 North America
12.2.1 U.S.
12.2.2 Canada
12.3 Europe
12.3.1 Germany
12.3.2 France
12.3.3 U.K.
12.3.4 Italy
12.3.5 Spain
12.3.6 Rest-of-Europe
12.4 Asia-Pacific
12.4.1 China
12.4.2 India
12.4.3 Japan
12.4.4 South Korea
12.4.5 Australia
12.4.6 Rest-of-Asia-Pacific
12.5 Rest-of-the-World (RoW)
13 Company Profiles
13.1 Agilent Technologies, Inc.
13.1.1 Company Overview
13.1.2 Role of Agilent Technologies, Inc. in the Global Molecular Oncology Diagnostics Market
13.1.3 Key Competitors of the Company
13.1.4 Business Strategies
13.1.4.1 Merger and Acquisition
13.1.4.2 Product Launch/Approval
13.1.4.3 Expansion
13.1.5 Financials
13.1.6 Analyst Perspective
13.2 Abbott.
13.2.1 Company Overview
13.2.2 Role of Abbott. in the Global Molecular Oncology Diagnostics Market
13.2.3 Key Competitors of the Company
13.2.4 Key Customers of the Company
13.2.5 Financials
13.2.6 Key Insights about the Financial Health of the Company
13.2.7 Analyst Perspective
13.3 Biocartis NV
13.3.1 Company Overview
13.3.2 Role of Biocartis NV in the Global Molecular Oncology Diagnostics Market
13.3.3 Key Competitors of the Company
13.3.4 Corporate Strategies
13.3.4.1 Synergistic Activities
13.3.5 Business Strategies
13.3.5.1 Product Launch/Approval
13.3.6 Financials
13.3.7 Analyst Perspective
13.4 Bio-Rad Laboratories, Inc.
13.4.1 Company Overview
13.4.2 Role of Bio-Rad Laboratories, Inc. in the Global Molecular Oncology Diagnostics Market
13.4.3 Key Competitors of the Company
13.4.4 Corporate Strategies
13.4.4.1 Synergistic Activities
13.4.5 Business Strategies
13.4.5.1 Merger and Acquisition
13.4.5.2 Product Launch/Approval
13.4.6 Financials
13.4.7 Analyst Perspective
13.5 F. Hoffmann-La Roche Ltd.
13.5.1 Company Overview
13.5.2 Role of F. Hoffmann-La Roche Ltd. in the Global Molecular Oncology Diagnostics Market
13.5.3 Key Competitors of the Company
13.5.4 Corporate Strategies
13.5.4.1 Synergistic Activities
13.5.5 Business Strategies
13.5.5.1 Product Launch/Approval
13.5.5.2 Expansion
13.5.6 Financials
13.5.7 Analyst Perspective
13.6 QIAGEN N.V.
13.6.1 Company Overview
13.6.2 Role of QIAGEN N.V. in the Global Molecular Oncology Diagnostics Market
13.6.3 Key Competitors of the Company
13.6.4 Key Customers of the Company
13.6.5 Corporate Strategies
13.6.5.1 Synergistic Activities
13.6.6 Business Strategies
13.6.6.1 Merger and Acquisition
13.6.6.2 Product Launch/Approval
13.6.7 Financials
13.6.8 Analyst Perspective
13.7 Thermo Fisher Scientific, Inc.
13.7.1 Company Overview
13.7.2 Role of Thermo Fisher Scientific, Inc. in the Global Molecular Oncology Diagnostics Market
13.7.3 Key Competitors of the Company
13.7.4 Corporate Strategies
13.7.4.1 Synergistic Activities
13.7.5 Business Strategies
13.7.5.1 Product Launch/Approval
13.7.5.2 Expansion
13.7.6 Financials
13.7.7 Analyst Perspective
13.8 Danaher.
13.8.1 Company Overview
13.8.2 Role of Danaher. in the Global Molecular Oncology Diagnostics Market
13.8.3 Key Competitors of the Company
13.8.4 Corporate Strategies
13.8.4.1 Synergistic Activities
13.8.5 Financials
13.8.6 Key Insights about the Financial Health of the Company
13.8.7 Analyst Perspective
13.9 Guardant Health
13.9.1 Company Overview
13.9.2 Role of Guardant Health in the Global Molecular Oncology Diagnostics Market
13.9.3 Key Competitors of the Company
13.9.4 Key Customers of the Company
13.9.5 Corporate Strategies
13.9.5.1 Synergistic Activities
13.9.6 Business Strategies
13.9.6.1 Product Launch/Approval
13.9.7 Financials
13.9.8 Key Insights about the Financial Health of the Company
13.9.9 Analyst Perspective
13.1 HTG Molecular Diagnostics, Inc.
13.10.1 Company Overview
13.10.2 Role of HTG Molecular Diagnostics, Inc. in the Global Molecular Oncology Diagnostics Market
13.10.3 Key Competitors of the Company
13.10.4 Business Strategies
13.10.4.1 Product Launch/Approval
13.10.5 Financials
13.10.6 Key Insights about the Financial Health of the Company
13.10.7 Analyst Perspective
13.11 Illumina, Inc.
13.11.1 Company Overview
13.11.2 Role of Illumina, Inc. in the Global Molecular Oncology Diagnostics Market
13.11.3 Key Competitors of the Company
13.11.4 Corporate Strategies
13.11.4.1 Synergistic Activities
13.11.5 Business Strategies
13.11.5.1 Merger and Acquisition
13.11.6 Financials
13.11.7 Key Insights about the Financial Health of the Company
13.11.8 Analyst Perspective
13.12 Invivoscribe, Inc.
13.12.1 Company Overview
13.12.2 Role of Invivoscribe, Inc. in the Global Molecular Oncology Diagnostics Market
13.12.3 Key Competitors of the Company
13.12.4 Corporate Strategies
13.12.4.1 Synergistic Activities
13.12.5 Business Strategies
13.12.5.1 Product Launch/Approval
13.12.6 Analyst Perspective
13.13 Myriad Genetics, Inc.
13.13.1 Company Overview
13.13.2 Role of Myriad Genetics, Inc. in the Global Molecular Oncology Diagnostics Market
13.13.3 Key Competitors of the Company
13.13.4 Corporate Strategies
13.13.4.1 Synergistic Activities
13.13.5 Business Strategies
13.13.5.1 Product Launch/Approval
13.13.6 Financials
13.13.7 Key Insights about the Financial Health of the Company
13.13.8 Analyst Perspective
13.14 Sysmex Corporation
13.14.1 Company Overview
13.14.2 Role of Sysmex Corporation in the Global Molecular Oncology Diagnostics Market
13.14.3 Key Competitors of the Company
13.14.4 Corporate Strategies
13.14.4.1 Synergistic Activities
13.14.5 Business Strategies
13.14.5.1 Product Launch/Approval
13.14.5.2 Expansion
13.14.6 Financials
13.14.7 Key Insights about the Financial Health of the Company
13.14.8 Analyst Perspective
List of Figures
Figure 1: Annual NIH Funding in Human Genomics Research, $Billion, FY2013-FY2019
Figure 2: Global Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 3: Global Molecular Oncology Diagnostics Market, Dynamics
Figure 4: Share of Key Market Strategies and Developments, January 2019-October 2022
Figure 5: Global Molecular Oncology Diagnostics Market (by Product), $Million, 2021 Vs. 2032
Figure 6: Global Molecular Oncology Diagnostics Market (by Technology), $Million, 2021 Vs. 2032
Figure 7: Global Molecular Oncology Diagnostics Market (by Application), $Million, 2021 Vs. 2032
Figure 8: Global Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021 Vs. 2032
Figure 9: Global Molecular Oncology Diagnostics Market (by End User), $Million, 2021 Vs. 2032
Figure 10: Global Molecular Diagnostics Market (by Region), $Million, 2021 Vs. 2032
Figure 11: Role of Diagnostics in Healthcare
Figure 12: Global Molecular Oncology Diagnostics Market Segments
Figure 13: Global Molecular Oncology Diagnostics Market: Methodology
Figure 14: Primary Research Methodology
Figure 15: Bottom-Up Approach (Segment-Wise Analysis)
Figure 16: Top-Down Approach (Segment-Wise Analysis)
Figure 17: Global Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 18: Solid Tumor Prevalence and Incidence Data in Europe, 2020
Figure 19: Hematological Malignancy Prevalence and Incidence Data in North America, 2020
Figure 20: Solid Tumor Prevalence and Incidence Data in Europe, 2020
Figure 21: Hematological Malignancy Prevalence and Incidence Data in Europe, 2020
Figure 22: Solid Tumor Prevalence and Incidence Data in Asia-Pacific, 2020
Figure 23: Hematological Malignancy Prevalence and Incidence Data in Asia-Pacific, 2020
Figure 24: Global Molecular Oncology Diagnostics Market, $Million, 2019-2021
Figure 25: Prominent FDA-Approved CDx Tests for Molecular Oncology Diagnostics
Figure 26: FDA Guidelines for CDx Approval
Figure 27: Criteria for CMS Coverage/Reimbursement
Figure 28: Europe In Vitro Diagnostic Devices Regulation Regulatory Process
Figure 29: Workflow for Medical Device Regulations
Figure 30: Global Molecular Diagnostics Market: Market Dynamics
Figure 31: Likert Scale
Figure 32: Impact Analysis of Market Drivers and Challenges on the Global Molecular Oncology Diagnostics Market
Figure 33: Global Distribution of Cases and Deaths (by Cancer Type), 2020
Figure 34: Global Incidence for Cancer Types (2017-2019)
Figure 35: Product Upgradations and Technological Advancements, 2019-2022
Figure 36: Synergistic Activities, 2019-2021
Figure 37: Share of Key Developments and Strategies, January 2019-October 2022
Figure 38: Share of Mergers and Acquisitions (by Company), January 2019-October 2022
Figure 39: Share of Synergistic Activities (by Company), January 2019-October 2022
Figure 40: Share of Product Launch and Approval Activities (by Company), January 2019-October 2022
Figure 41: Share of Expansion, Insurance, and Other Key Developments (by Company), January 2019-October 2022
Figure 42: Market-Share Analysis for the Global Molecular Oncology Diagnostics Market, $Million, 2021
Figure 43: Growth-Share Analysis of Global Molecular Oncology Diagnostics Market (by Technology), 2021-2032
Figure 44: Global Molecular Oncology Diagnostics Market (by Product)
Figure 45: Share of Global Molecular Oncology Diagnostics Market (by Product), $Million, 2021 and 2032
Figure 46: Global Molecular Oncology Diagnostics Market (Kits and Assays), $Million, 2021-2032
Figure 47: Global Molecular Oncology Diagnostics Market (Instruments), $Million, 2021-2032
Figure 48: Global Molecular Oncology Diagnostics Market (Software), $Million, 2021-2032
Figure 49: Global Molecular Oncology Diagnostics Market (by Technology)
Figure 50: Share of Global Molecular Oncology Diagnostics Market (by Technology), $Million, 2021 and 2032
Figure 51: Global Molecular Oncology Diagnostics Market (Polymerase Chain Reaction), $Million, 2021-2032
Figure 52: Global Molecular Oncology Diagnostics Market (Next-Generation Sequencing), $Million, 2021-2032
Figure 53: Global Molecular Oncology Diagnostics Market (Immunohistochemistry), $Million, 2021-2032
Figure 54: Global Molecular Oncology Diagnostics Market (Fluorescence In-Situ Hybridization), $Million, 2021-2032
Figure 55: Global Molecular Oncology Diagnostics Market (Flow Cytometry), $Million, 2021-2032
Figure 56: Global Molecular Oncology Diagnostics Market (Other Technologies), $Million, 2021-2032
Figure 57: Share of Global Molecular Oncology Diagnostics Market (by Application), $Million, 2021 and 2032
Figure 58: Global Molecular Oncology Diagnostics Market (Clinical Diagnostic), $Million, 2021-2032
Figure 59: Global Molecular Oncology Diagnostics Market (Research Use), $Million, 2021-2032
Figure 60: Global Molecular Oncology Diagnostics Market (by Cancer Type)
Figure 61: Share of Global Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021 and 2032
Figure 62: Global Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021 and 2032
Figure 63: Global Molecular Oncology Diagnostics Market (Breast Cancer), $Million, 2021-2032
Figure 64: Global Molecular Oncology Diagnostics Market (Lung Cancer), $Million, 2021-2032
Figure 65: Global Molecular Oncology Diagnostics Market (Colorectal Cancer), $Million, 2021-2032
Figure 66: Global Molecular Oncology Diagnostics Market (Prostate Cancer), $Million, 2021-2032
Figure 67: Global Molecular Oncology Diagnostics Market (Ovarian Cancer), $Million, 2021-2032
Figure 68: Global Molecular Oncology Diagnostics Market (Other Solid Tumors), $Million, 2021-2032
Figure 69: Global Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021 and 2032
Figure 70: Global Molecular Oncology Diagnostics Market (Lymphoma), $Million, 2021-2032
Figure 71: Global Molecular Oncology Diagnostics Market (Leukemia), $Million, 2021-2032
Figure 72: Global Molecular Oncology Diagnostics Market (Multiple Myeloma), $Million, 2021-2032
Figure 73: Global Molecular Oncology Diagnostics Market (Other Hematological Malignancies), $Million, 2021-2032
Figure 74: Share of Global Molecular Oncology Diagnostics Market (by End User), $Million, 2021 and 2032
Figure 75: Global Molecular Oncology Diagnostics Market (Hospitals and Diagnostic Centers), $Million, 2021-2032
Figure 76: Global Molecular Oncology Diagnostics Market (Reference Laboratories), $Million, 2021-2032
Figure 77: Global Molecular Oncology Diagnostics Market (Pharmaceutical and Biotechnology Companies), $Million, 2021-2032
Figure 78: Global Molecular Oncology Diagnostics Market (Academic and Research Institutes), $Million, 2021-2032
Figure 79: Global Molecular Oncology Diagnostics Market Snapshot (by Region), $Million, 2021-2032
Figure 80: Global Molecular Oncology Diagnostics Market (by Region), $Million, 2021-2032
Figure 81: North America Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 82: North America: Market Dynamics
Figure 83: North America Molecular Oncology Diagnostics Market (by Country), $Million, 2021 and 2032
Figure 84: U.S. Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 85: U.S. Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 86: U.S. Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 87: U.S. Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 88: Canada Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 89: Canada Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 90: Canada Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 91: Canada Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 92: Europe Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 93: Europe: Market Dynamics
Figure 94: Europe Molecular Oncology Diagnostics Market (by Country), $Million, 2021 and 2032
Figure 95: Germany Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 96: Germany Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 97: Germany Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 98: Germany Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 99: France Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 100: France Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 101: France Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 102: France Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 103: U.K. Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 104: U.K. Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 105: U.K. Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 106: U.K. Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 107: Italy Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 108: Italy Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 109: Italy Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 110: Italy Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 111: Spain Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 112: Spain Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 113: Spain Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 114: Spain Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 115: Rest-of-Europe Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 116: Rest-of-Europe Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 117: Rest-of-Europe Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 118: Rest-of-Europe Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 119: Asia-Pacific Molecular Oncology Diagnostics Market (by Region), $Million, 2021-2032
Figure 120: Asia-Pacific: Market Dynamics
Figure 121: Asia-Pacific Molecular Oncology Diagnostics Market (by Country), $Million, 2021 and 2032
Figure 122: China Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 123: China Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 124: China Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 125: China Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 126: India Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 127: India Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 128: India Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 129: India Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 130: Japan Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 131: Japan Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 132: Japan Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 133: Japan Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 134: South Korea Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 135: South Korea Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 136: South Korea Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 137: South Korea Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 138: Australia Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 139: Australia Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 140: Australia Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 141: Australia Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 142: Rest-of-Asia-Pacific Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 143: Rest-of-Asia-Pacific Molecular Oncology Diagnostics Market (by Cancer Type), $Million, 2021-2032
Figure 144: Rest-of-Asia-Pacific Molecular Oncology Diagnostics Market (by Solid Tumor), $Million, 2021-2032
Figure 145: Rest-of-Asia-Pacific Molecular Oncology Diagnostics Market (by Hematological Malignancy), $Million, 2021-2032
Figure 146: Rest-of-the-World Molecular Oncology Diagnostics Market, $Million, 2021-2032
Figure 147: Total Number of Companies Profiled
Figure 148: Agilent Technologies, Inc.: Product Portfolio
Figure 149: Agilent Technologies, Inc.: Overall Financials, $Million, 2019-2021
Figure 150: Agilent Technologies, Inc.: Revenue (by Segment), $Million, 2019-2021
Figure 151: Agilent Technologies, Inc.: Revenue (by Region), $Million, 2019-2021
Figure 152: Agilent Technologies, Inc: R&D Expenditure, $Million, 2019-2021
Figure 153: Abbott.: Overall Product Portfolio
Figure 154: Abbott.: Overall Financials, $Million, 2019-2021
Figure 155: Abbott.: Revenue (by Segment), $Million, 2019-2021
Figure 156: Abbott.: Revenue (by Region), $Million, 2019-2021
Figure 157: Abbott.: R&D Expenditure, $Million, 2019-2021
Figure 158: Biocartis NV.: Product Portfolio
Figure 159: Biocartis NV: Overall Financials, $Million, 2019-2021
Figure 160: Bio-Rad Laboratories, Inc.: Product Portfolio
Figure 161: Bio-Rad Laboratories, Inc.: Overall Financials, $Million, 2019-2021
Figure 162: Bio-Rad Laboratories, Inc.: Revenue (by Segment), $Million, 2019-2021
Figure 163: Bio-Rad Laboratories, Inc.: Revenue (by Region), $Million, 2019-2021
Figure 164: Bio-Rad Laboratories, Inc.: R&D Expenditure, $Million, 2019-2021
Figure 165: F. Hoffmann-La Roche Ltd.: Product Portfolio
Figure 166: F. Hoffmann-La Roche Ltd.: Overall Financials, $Million, 2019-2021
Figure 167: F. Hoffmann-La Roche Ltd.: Revenue (by Segment), $Million, 2019-2021
Figure 168: F. Hoffmann-La Roche Ltd.: Revenue (by Region), $Million, 2019-2021
Figure 169: F. Hoffmann-La Roche Ltd.: R&D Expenditure, $Million, 2019-2021
Figure 170: QIAGEN N.V.: Product Portfolio
Figure 171: QIAGEN N.V.: Overall Financials, $Million, 2019-2021
Figure 172: QIAGEN N.V.: Revenue (by Segment), $Million, 2019-2021
Figure 173: QIAGEN N.V.: Revenue (by Region), $Million, 2019-2021
Figure 174: QIAGEN N.V.: R&D Expenditure, $Million, 2019-2021
Figure 175: Thermo Fisher Scientific, Inc.: Product Portfolio
Figure 176: Thermo Fisher Scientific, Inc.: Overall Financials, $Million, 2019-2021
Figure 177: Thermo Fisher Scientific, Inc.: Revenue (by Segment), $Million, 2019-2021
Figure 178: Thermo Fisher Scientific, Inc.: Revenue (by Region), $Million, 2019-2021
Figure 179: Thermo Fisher Scientific, Inc.: R&D Expenditure, $Million, 2019-2021
Figure 180: Danaher.: Product Portfolio
Figure 181: Danaher.: Overall Financials, $Million, 2019-2021
Figure 182: Danaher.: Revenue (by Segment), $Million, 2019-2021
Figure 183: Danaher.: Revenue (by Region), $Million, 2019-2021
Figure 184: Danaher.: R&D Expenditure, $Million, 2019-2021
Figure 185: Guardant Health: Overall Product Portfolio
Figure 186: Guardant Health: Overall Financials, $Million, 2019-2021
Figure 187: Guardant Health: Revenue (by Segment), 2019-2021
Figure 188: Guardant Health: R&D Expenditure, $Million, 2019-2021
Figure 189: HTG Molecular Diagnostics, Inc.: Overall Product Portfolio
Figure 190: HTG Molecular Diagnostics, Inc.: Overall Financials, $Million, 2019-2021
Figure 191: HTG Molecular Diagnostics, Inc.: Revenue (by Segment), 2019-2021
Figure 192: HTG Molecular Diagnostics, Inc.: R&D Expenditure, $Million, 2019-2021
Figure 193: Illumina, Inc.: Overall Product Portfolio
Figure 194: Illumina, Inc.: Overall Financials, $Million, 2019-2021
Figure 195: Illumina, Inc.: Revenue (by Segment), $Million, 2019-2021
Figure 196: Illumina, Inc.: Revenue (by Region), $Million, 2019-2021
Figure 197: Illumina, Inc.: R&D Expenditure, $Million, 2019-2021
Figure 198: Invivoscribe, Inc.: Overall Product Portfolio
Figure 199: Myriad Genetics, Inc.: Product Portfolio
Figure 200: Myriad Genetics, Inc.: Overall Financials, $Million, 2019-2021
Figure 201: Myriad Genetics, Inc.: Revenue (by Segment), $Million, 2019-2021
Figure 202: Myriad Genetics, Inc.: R&D Expenditure, $Million, 2019-2021
Figure 203: Sysmex Corporation: Overall Product Portfolio
Figure 204: Sysmex Corporation: Overall Financials, $Million, 2019-2021
Figure 205: Sysmex Corporation: Revenue (by Region), $Million, 2019-2021
Figure 206: Sysmex Corporation: R&D Expenditure, $Million, 2019-2021
List of Tables
Table 1: Biomarkers for Different Cancer Types
Table 2: Cost of Liquid Biopsy Based NGS Diagnostic Kits
Table 3: Companies Providing Kits and Assays for Molecular Oncology Diagnostics
Companies Mentioned
Agilent Technologies, Inc.
Abbott.
Biocartis NV
Bio-Rad Laboratories, Inc.
F. Hoffmann-La Roche Ltd.
QIAGEN N.V.
Thermo Fisher Scientific, Inc.
Danaher.
Guardant Health
HTG Molecular Diagnostics, Inc.
Illumina, Inc.
Invivoscribe, Inc.
Myriad Genetics, Inc.
Sysmex Corporation




Reviews
There are no reviews yet.